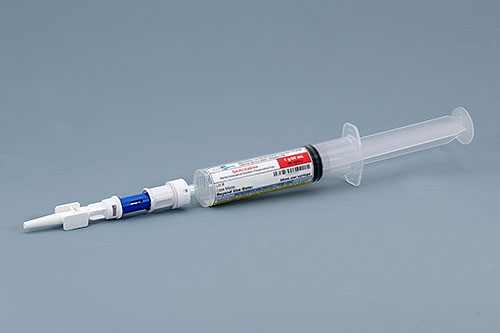
Edge Pharma offers compounded Gemcitabine Bladder Instillation Syringe kits that include a 1g / 50mL pre-filled unit-dose syringe of Gemcitabine, a closed system transfer device (CSTD), and catheter tip. The CSTD is attached by Edge in an ISO 5 environment according to cGMP. This solution solves the USP 800 hazardous compounding challenge many hospitals and clinics now face when trying to administer Gemcitabine. Since the product comes ready-to-administer, you do not need to compound a hazardous drug in your facility. Edge specializes in offering hazardous compounding services for organizations, making the transition to USP 800 compliance easier.
Gemcitabine Bladder Instillation Kit
Gemcitabine intravesical solution, sterile, preservative free.
Available Dosage=1 g
Syringe Volume=50 mL
Closed system transfer device (CSTD) and catheter tip included with kit.
While Gemcitabine is approved by the FDA for the treatment of ovarian, breast, cancer, lung, and pancreatic cancer, it is widely used “off label” in the treatment of bladder cancer.[1] From the FDA perspective, healthcare providers may prescribe a drug for an off label use if they deem it medically appropriate for their patient.[2]
A landmark national study, with 23 cancer centers participating, showed Gemcitabine’s great promise for bladder cancer treatment. The study was led by the Wilmot Cancer Institute at the University of Rochester. For the study, patients had their bladder tumor removed and immediately, post-surgery, were administered Gemcitabine through a catheter, directly into the bladder area.[3] The study showed a 34 percent reduction in cancer recurrence.[4]
Most bladder cancers – seven out of 10 cases – are diagnosed at an early stage when they are highly treatable, according to the Mayo Clinic. But even when treated, bladder cancer often recurs. “For this reason, people with bladder cancer typically need follow-up tests for years after treatment to look for bladder cancer that recurs or advances to a higher stage.”[5]
Each year in the U.S., some 56,000 men and 18,000 women are diagnosed with bladder cancer, according to the Centers for Disease Control.[6] Bladder cancer is the fourth most common cancer found in men in the U.S. – the chance a man will develop bladder cancer during his lifetime is about one in 27; the rate for women is about one in 89.[7]
[1] National Cancer Institute, online article, Gemcitabine Hydrochloride https://www.cancer.gov/
[2] U.S. Food and Drug Administration, online article, Understanding Unapproved Use of Approved Drugs “Off Label” https://www.fda.gov/
[3] Wilmot Cancer Institute, University of Rochester, online article, Investigators Looking to a Better Future for Patients with Bladder Cancer https://www.urmc.rochester.edu/
[4] Journal of the American Medical Association, online article, Effect of Intravesical Instillation of Gemcitabine vs. Saline Immediately following Resection of Suspected Low-Grade Non-Muscle-Invasive Bladder Cancer on Tumor Recurrence https://jamanetwork.com/
[5] Mayo Clinic, online article, Bladder Cancer: Symptoms and Causes https://www.mayoclinic.org/
[6] Centers for Disease Control and Prevention, online article, Bladder Cancer https://www.cdc.gov/
[7] American Cancer Society, online article, Key Statistics for Bladder Cancer https://www.cancer.org/
Call (802-992-1178) or email us (sales@edgepharma.com) to learn more about this great new product and to have it added to your online ordering account. We are now taking preorders!
Register To Order Online
We are currently licensed to sell to all US states except Alabama and Virginia. Please fill the form below to create an account. Medical, DEA, or hospital pharmacy licenses will be required to order gemcitabine.
Create an account to order
Log-In if you have an account

