Emergency Gynecologic Methotrexate Kit
The Emergency Gynecologic Methotrexate injection (mtx injection) from Edge Pharma solves a major issue affecting hospitals and OB/GYN clinics. In facilities without a USP 800 compliant hazardous cleanroom, preparation and administration of Methotrexate is a challenge. With the EmGyn Emergency Gynecologic Methotrexate Kit, any hospital or clinic can now confidently administer adjustable patient-specific body surface area based doses of Methotrexate up to 125mg. As an FDA-Registered 503B Outsourcing Facility we are able to dispense EmGyn Kits without requiring patient names.
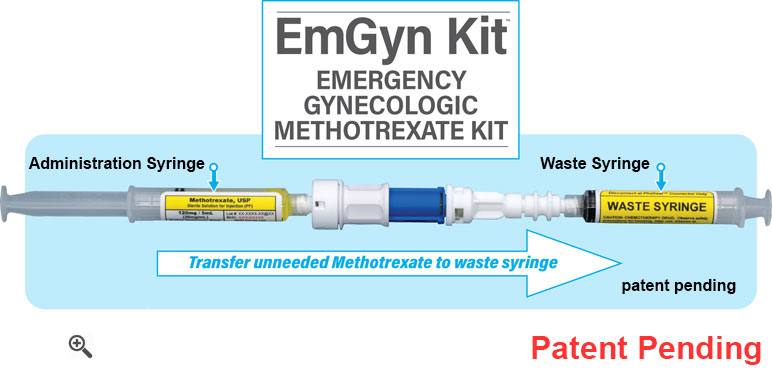
Emergency Gynecologic Methotrexate Kit comes prefilled with 5mL of Methotrexate 25 mg/mL in an administration syringe. The administration syringe is connected to a waste syringe through a closed system transfer device (CSTD). Body surface area based dosage is achieved by activating the CSTD, transferring the unneeded Methotrexate into the waste syringe, deactivating the CSTD, and then detaching the waste syringe from the system. The waste syringe can then be safely disposed of in an appropriate hazardous waste container, while the administration syringe now contains the desired amount of Methotrexate. Hence any dose up to 125mg can be selected and easily prepared for administration.
EmGyn Kit Features
- The simplest solution for Emergency Methotrexate to help comply with USP 800.
- 125mg/5mL Methotrexate. Enables body surface area based dosing.
- Ability to prepare any dosage up to 125mg without the need for hazardous cleanroom facilities. No compounding required.
- Dispensed without Patient Names.
- Includes closed system transfer device.
 EmGyn Kit Documentation (pdf)
EmGyn Kit Documentation (pdf)
An ectopic pregnancy is when a fertilized egg grows outside the uterus, usually in a fallopian tube.(1) The March of Dimes reports that 1 in 50 pregnancies is ectopic, or “tubal.” Ectopic pregnancy always ends in pregnancy loss.(1) The American College of Obstetricians and Gynecologists lists several risk factors for ectopic pregnancy. They include:
• Previous ectopic pregnancies
• Prior fallopian tube surgery
• Previous pelvic or abdominal surgery
• Certain sexually transmitted diseases
• Pelvic inflammatory disease
• Endometriosis
• Cigarette smoking
• Age older than 35 years(2)
While initially an ectopic pregnancy may seem like a typical pregnancy, particular symptoms indicate the pregnancy may be ectopic. These include abnormal bleeding, low back pain, and cramping on one side of the pelvis. As the pregnancy grows, more serious symptoms may be experienced such as sharp pain and fainting. A ruptured fallopian tube can cause bleeding that is life threatening to the mother.(3)
An ectopic pregnancy is diagnosed by a pelvic exam, an ultrasound and blood tests. An early ectopic pregnancy is most often treated with methotrexate, according to the Mayo Clinic. Methotrexate works by dissolving cells and preventing new cell growth.(4) The pregnancy is absorbed by the body and removal of the fallopian tube is not required.(5)
(1) March of Dimes, online article, Ectopic Pregnancy https://www.marchofdimes.org/
(2) American College of Obstetricians and Gynecologists, online article, FAQ Ectopic Pregnancy https://www.acog.org/
(3) American College of Obstetricians and Gynecologists, online article, FAQ Ectopic Pregnancy https://www.acog.org/
(4) Mayo Clinic, online article, Ectopic Pregnancy: Diagnosis and Treatment https://www.mayoclinic.org/
(5) American College of Obstetricians and Gynecologists, online article, FAQ Ectopic Pregnancy https://www.acog.org/
References
Cecchino, G.N., Araujo Júnior, E. & Elito Júnior, “Methotrexate for Ectopic Pregnancy: When and How,” J. Arch Gynecol Obstet (2014) 290: 417. https://doi.org/
“In spite of new treatment modalities and improvement of diagnostic methods, ectopic pregnancy (EP) is still the leading cause of maternal death in the first trimester of pregnancy, accounting for 6-13% of all pregnancy-related deaths.”
“In industrialized countries, up to 2% of all pregnancies are ectopic in location.
“Considering MTX in tubal pregnancy, it proved to be more effective in cases of low titers of beta-hCG and masses with a small diameter, although there is still no uniformity of these parameters. Additionally, non-tubal ectopic pregnancies are associated with greater morbidity and may require medical treatment with direct injection or systemic MTX, which have been used effectively.”
Register To Order Online
We are currently licensed to sell to all US states except Alabama and Virginia. Register for an online ordering account to order mtx injection.
Create an account to order
Log-In if you have an account

